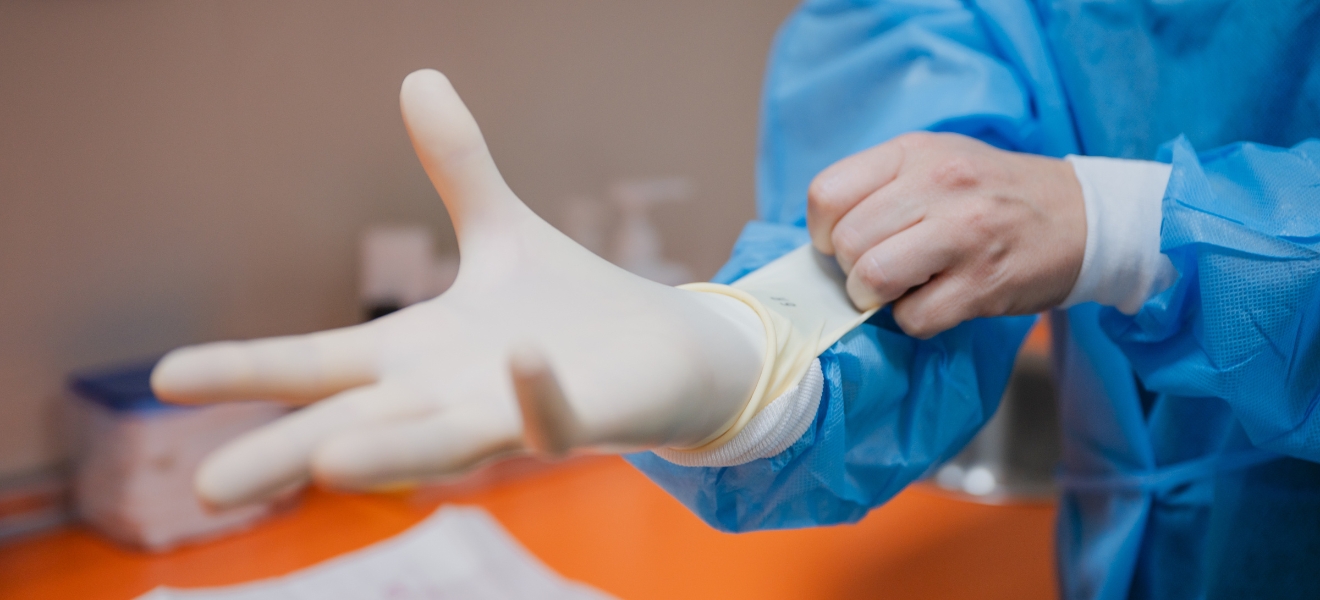
Guiding pharmaceutical manufacturers in cleanroom gloves selection
Cleanroom gloves are a critical—yet often underestimated—control within pharmaceutical contamination control strategies.
This article proposes a practical, risk-based approach to glove selection that balances performance, regulatory compliance, operator safety, and sustainability. It covers key decision criteria such as material compatibility, barrier integrity, particulate and microbial control, ergonomics, and lifecycle impact. The article also explains how glove choices influence aseptic behavior, environmental monitoring outcomes, and overall process robustness. By aligning glove strategy with EU GMP expectations and real operational constraints, manufacturers can reduce contamination risk, improve operator performance, and support long-term compliance through informed, sustainable decisions.
Overview
- Define intended use and critical risks (process, grade, interventions, product exposure).
- Select materials and designs based on compatibility, integrity performance, and ergonomics.
- Align glove strategy with EU GMP/Annex 1 expectations, EM approach, and CCS rationale.
- Integrate lifecycle thinking (supply security, waste, sustainability) without compromising sterility assurance.
Find a way to help align selection criteria with real operational constraints and quality objectives. Outcome: an informed glove strategy that improves control, consistency, and long-term compliance.